Abstract
Background:
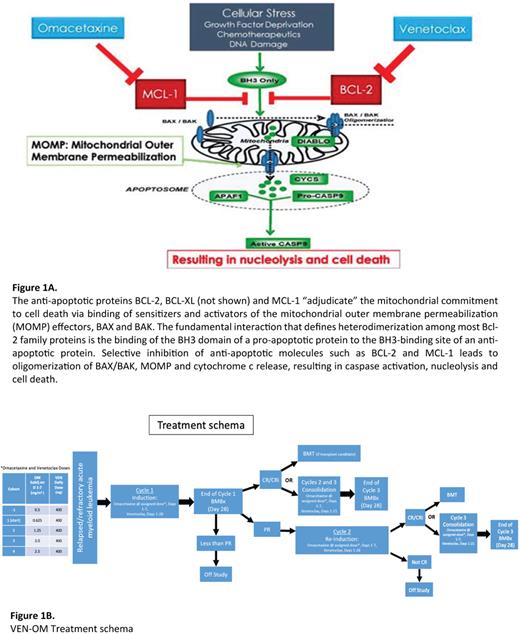
Venetoclax (VEN) combined with hypomethylating agents (HMA) are increasingly used as first line (1L) treatment in AML patients (pt) intolerant of intensive chemotherapy, due to the high complete remission (CR) rate (66.4%), tolerance of therapy and a median duration CR of 17.5 months. However, >60% of pts discontinued VEN/HMA (n=286) during the VIALE-A trial, mostly due to disease progression (DiNardo et al.N Engl J Med2020; 383:617-29). Outcomes for such pts receiving 2L and 3L therapies for relapsed/refractory (R/R) AML are poor and may be due to development of resistance to BCL-2 inhibition through mechanisms including the upregulation of the anti-apoptotic proteins BCL-XL or MCL1. In vitro studies demonstrate that VEN-resistant leukemia stem cells (LSC) are re-sensitized to BCL-2 inhibition when MCL-1 is also inhibited (Ramsey et al. Cancer Discov 2018; 8:1566-81). Novel MCL-1 inhibitors are thus in phase 1 clinical trials in R/R AML (e.g. NCT02675452), however there have been concerns for cardiotoxicity. Metabolic studies (Stevens et al. Nat Commun 2018: 9, 3694) indicate that the CD123+ malignant stem cell compartment of pts with high-grade MDS rely on upregulation of the protein synthesis machinery and mitochondrial oxidative phosphorylation (OxPhos). Omacetaxine (OM), a protein translation inhibitor both inhibits OxPhos and down-regulates (other) proteins with a short half-life such as MCL1. Treatment of a xenograft AML model with a combination of OM and VEN indicates in vivo synergistic effects in impairing mitochondrial respiration, ATP production, BCL-2 inhibition and MCL-1 downregulation (Fig 1A). We hypothesize this combination will prove effective in pts with R/R AML including those resistant to VEN-containing regimens. A similar drug combination is being studied by Dinardo et al. in R/R AML and MDS pts with RUNX1 mutations.
Methods:
VEN-OM is a single arm, open-label, phase Ib dose-escalation study in R/R AML pts (18-75y). The trial utilizes an innovative Bayesian Optimal Interval (BOIN) adaptive design (Yuan et al. Clin Cancer Res 2016)to determine the maximum tolerated dose (MTD), with a target dose-limiting toxicity (DLT) rate of 2%, and 4 pre-specified dose levels of OM, involving 24-30 pts.
Major eligibility: ECOG 0-2, adequate organ function, R/R AML having progressed on ≥ 1L of therapy, which must have included a VEN-containing regimen. Exclusion criteria: Prior use of OM, peripheral blast count > 25 × 109/L, unresolved ≥ grade 2 clinically significant nonhematologic toxicities from prior anticancer therapy, unresolved ≥ grade 2 disseminated intravascular coagulation (DIC), other active malignancy within 1 year before study entry, or active infections.
Induction Phase: Beginning with the first pt treated at the lowest dose of OM (0.625 mg/m2 q12h), OM dose escalation and de-escalation will involve comparison of the observed DLT rate at the current dose with a pair of fixed, pre-specified boundary rates. After a VEN ramp-up phase (days 1-3), the VEN dose is fixed at 400mg daily on days 4-28. OM doses are given days 1-7. OM dose will be escalated for each subsequent pt until the first DLT occurs, whereupon the current dose cohort is expanded to 3. In simulations of these conditions with a target DLT rate of 20%, the percentage correct selection of the MTD is between 60% with enrollment of 20 pts and 70% with 24 pts.
Consolidation Phase: OM dose will be as per induction dose for days 1-7 of each cycle. VEN dose is 400mg days 1-21. Regimen comprises 3 cycles: Induction cycle x 1 and Consolidation cycles x 2 (Fig 1B). Primary endpoint is MTD of the combination of VEN and OM. Secondary endpoints include overall response rate after 3 cycles, characterization of adverse effects, and estimates of EFS and OS at 12 months. Exploratory objectives include measurement of validated markers of early renal injury including urine TIMP-2 x IGFBP7 and KIM-1 to identify pts susceptible to OM-induced nephrotoxicity and potential correlation with OM efficacy. Our previous experience with OM added to 7+3 ("AML-02", ASH 2022 abstract submitted) suggests that quantification of BM leukemia blast protein synthesis rates (Walker et al. Clin Cancer Res 2021, 27:819-30) and 'BH3 profiling' at presentation predicts outcomes, and these correlative studies will also be performed. The study recently opened, with the first patient completing induction without complications. Trial registered as (NCT04926285).
Disclosures
Arain:Astellas Pharma: Current Employment. Patel:Exelixis: Current Employment. Calip:Flatiron Health, Inc.: Current Employment; Pfizer: Research Funding; Roche: Current equity holder in publicly-traded company. Quigley:Alnylam: Speakers Bureau; Servier: Speakers Bureau; Agios: Speakers Bureau; Rigel: Other: Advisory Board.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal